Zyban tablets instructions for use, reviews, analogues, side effects
Zyban is an atypical antidepressant used to treat emotional-volitional disorders. Another point of application of Zyban is the treatment of nicotine addiction. Thanks to a special mechanism of action on the central nervous system, the drug helps to relieve smoking cessation.
Form of production
Zyban is manufactured and available in tablet form by the British company GlaxoSmithKline. The standard dosage is 150 mg of the active substance in each tablet. In a blister of 10 tablets, in a package of 3 to 12 blisters.

Composition
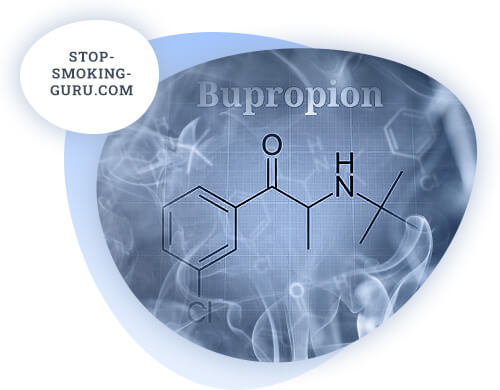
The main active ingredient of Zyban is the antidepressant bupropion. Each tablet contains 150 mg of bupropion. In addition to the active ingredient, the remedy contains a number of auxiliary components. Their concentrations in each tablet are very low, so they have no effect on the body.
Pharmacological group
Zyban by its main active substance belongs to the group of atypical antidepressants. These are drugs that have a psychotropic effect with an effect on the emotional-volitional sphere. The zyban subgroup is selective inhibitors of dopamine and norepinephrine reuptake. This subgroup includes a very limited number of drugs.
Pharmacological action
The pharmacological action of zyban is due to the effect of bupropion on the body. Bupropion is not homogeneous in its composition. In the body, it breaks down into several metabolites, each of which has its own effect on the cells of the central nervous system.
Bupropion is a selective inhibitor of norepinephrine and dopamine reuptake. This means that the drug prevents these mediators from entering neurons from the synaptic cleft. At the same time, the concentration of these substances increases and they bind more frequently to the receptors of neurons, which are also located in the synaptic cleft. The more dopamine and norepinephrine affect the receptors, the better the patient feels.
The development of depression is associated with a decrease in serotonin, norepinephrine, and dopamine in the brain. Bupropion provides an increase in the concentration of the latter two mediators, which contributes to the improvement of mood. However, this is not all of the drug's effects.
By acting on dopamine receptors, bupropion reduces the feeling of hunger and allows to fight obesity more effectively. In addition, the substance helps to cope with nicotine addiction. Bupropion is able to bind to nicotine receptors, thus tricking the brain into quitting smoking tobacco.
Indications
There have been many studies determining the effectiveness of zyban in the treatment of a particular pathological condition. As a result, diseases in which it is advisable to prescribe this drug have been identified. These conditions include:
- A first episode of mild to moderate depression;
- Recurrent depressive disorder;
- Bipolar affective disorder, current episode of depression;
- Nicotine addiction;
- Obesity;
- Decreased libido, including that caused by other antidepressants;
- Attention deficit hyperactivity disorder in adults.
For other psychiatric disorders, the effectiveness of zyban has not been proven. For example, a study conducted in relation to anxiety reduction has shown that zyban does not have an anti-anxiety component. However, in some cases, it can be used for social phobias.
Contraindications
Zyban should not be prescribed for the following conditions:
- Epilepsy;
- Nervous tics, including Tourette's syndrome;
- Benzodiazepine tranquilizer withdrawal syndrome;
- Eating disorders;
- Cancer of the brain;
- Third-degree arterial hypertension;
- Renal or hepatic insufficiency in the decompensation stage;
- Childhood and adolescence.
In addition, taking zyban with monoamine oxidase inhibitors is prohibited. There should be a gap of two weeks between the doses of these drugs.
Side effects
The side effects of zyban mainly concern the nervous system. The most common are epileptic seizures, tics and hand tremors. The drug is especially dangerous for patients who already have seizure readiness or other neurological disorders.
Another frequent complication is headache, dizziness, and nausea. These symptoms develop during the first days of taking the drug and soon disappear. About 1% of drug users may develop arterial hypertension, most frequently its isolated systolic variant. It does not matter whether the patient had this disease before the start of therapy or not.
A rare side effect of zyban is galactorrhea, the secretion of milk from the mammary glands outside of pregnancy or lactation. Another rare complication of treatment with this drug is toxic liver damage.
With long-term use of Zyban and abrupt discontinuation of treatment, withdrawal syndrome is possible. Other side effects are currently unknown, post-marketing studies of the drug are underway to expand this list.
Instructions for use
It is recommended to use Zyban twice a day. The half-life of the drug is less than 24 hours, so it is important to divide the daily dose into two doses 12 hours apart (see chart). You should start treatment with the minimum dosage of 300 mg. It is enough to take one tablet in the morning and one in the evening.
Within two weeks, the dose can be increased to 450 mg per day. You can take two tablets in the morning and one in the evening, or two in the evening and one in the morning. It is also possible to divide the tablet and drink one and a half in the morning and one and a half in the evening.
The dosage can be increased again after another two weeks if necessary. The maximum permitted amount of the drug used per day is 600 mg. This should not be exceeded because of the risk of side effects.
The drug can be taken regardless of meals. The tablets should be swallowed with a small amount of water.
The drug does not affect the sleep and wakefulness mode. Restriction in driving any modes of transport is not required. There are data about the development of dependence on Zyban and the occurrence of withdrawal syndrome after stopping its use. To avoid the risk of developing this syndrome, you should stop taking it gradually. You should start by reducing the dosage by 150 mg once every two weeks until you have completely stopped the drug.
Analogues
Analogues of the drug, which have the same active substance, are:
- Wellbutrin;
- Voxra;
- Budeprion;
- Aplensin.
Reviews
Kirill N.: "Took Zyban while quitting smoking. At first I didn't notice the effect. I wanted to smoke the same way as before, even irritability increased a little. But I only had to keep it up for a week, then the effect of the drug appeared. Usually this is the hardest time, but Ziban helped me to overcome it easily. I practically didn't want to smoke, so I quit after all."
Psychiatrist: "The use of Zyban in our country is very limited. It is a new drug that has a mechanism of action that differs from other drugs. It is still poorly studied and its prescription raises some concerns for the health of patients. Moreover, the cost of the drug does not allow its use for treatment of most patients. At the same time, American colleagues are successfully introducing zyban into their practice, so we can expect its distribution in the domestic market of pharmaceuticals in the near future."
Does zyban pose a threat to the patient?
On February 4, 2002, in the meeting room of the Scientific Council of the Institute of Cardiology named after N.D. Ammosov. N.D. The press-conference devoted to the questions of clinical application of Zyban (bupropion hydrochloride) for the nicotine addiction elimination was held on the initiative of the head of Countrywide Integrated Noncommunicable Diseases Intervention Program (CINDI - Ukraine), Doctor of Medical Science, professor Irina Smirnova and the head of the State Pharmacological Center, the head of the clinical pharmacology department of the D, Professor Alexey Victorov, M.D., with the support of GlaxoSmithKline Pharmaceuticals. The press-conference was conducted by Alexander Voloshin, the head of press-centre of the Ministry of Healthcare of Ukraine.
On January 14, 2002 the official site www.mca.gov.uk of UK Medical Controls Agency (MCA) had information about adverse reactions to Zyban (bupropion hydrochloride) used in Great Britain to treat nicotine addiction, i.e. as a medicine that helps to quit smoking. In 2001, the drug was registered in Ukraine. Among other things, the document "Zyban (bupropion hydrochloride) - safety update" ("Zyban (bupropion hydrochloride) - safety analysis") reported about 57 deaths of patients who took Zyban for smoking cessation. At the same time, it was noted that "a causal link between the use of Zyban and the deaths was not established, and in most cases the alternative explanation (of the fact of death. - Author's note) is the patient's underlying disease. In 14 reports, there were indications that the patients were not taking Zyban before they died. Unfortunately, some media outlets have acted like a "spoiled telephone", reporting only half the truth - about the death of 57 patients who had taken Zyban, while "forgetting" to specify the important circumstance that the official MCA document states - the lack of a credible link between taking Zyban and the death of patients. Of course, the MCA made the appropriate decision to continue to monitor the safety of Zyban, drawing the attention of practitioners to the need to consider the contraindications listed in the instructions for its use when prescribing this drug. In addition, the MCA specifically highlighted one of the contraindications to the use of Zyban - the presence of current or history of seizures.

The MCA document mentioned above states that Zyban was registered in the United Kingdom in June 2000. From the time the drug was licensed in that country until November 30, 2001, about 500,000 patients with nicotine addiction had already used it. The drug proved to be an effective and safe remedy. This is also evidenced by the significant experience of its clinical use in the United States and Canada for some depressive conditions. In the U.S., Zyban was registered as far back as 1989, at first as a treatment for depression. Since that time, 28 million patients around the world have used it (20 million for various forms of depression and 8 million for nicotine addiction).
As noted by Irina Smirnova, about 50% of men of working age in Ukraine abuse tobacco.
There is an unfavorable tendency of smoking prevalence increase among young people as well as women. To date, proven harm of passive smoking. Especially dangerous is the impact of tobacco smoke on children's health.
Smoking is a risk factor for many chronic diseases, especially cardiovascular diseases. In Ukraine in the structure of causes of mortality cardiovascular diseases account for 60.3%. Moreover, smoking is also the cause of serious respiratory, digestive and other system diseases. At the same time 80% of malignant neoplasms occur with abusers of tobacco.
Over time, tobacco smokers develop nicotine addiction. About 90% of smokers say they want to quit. However, only 3-5% of people succeed. Therefore, an important practical problem that physicians need to address is helping smokers overcome nicotine addiction and stop smoking. Currently, experts consider nicotine addiction as a disease. An important role in alleviating the symptoms of nicotine addiction is played by pharmacological means. There are two trends in pharmacotherapy of nicotine addiction - the use of Zyban (bupropion hydrochloride) GlaxoSmithKline, which affects directly the subtle biochemical mechanisms of nicotine addiction formation in certain structures of the brain, and nicotine replacement therapy, which results in a basal concentration of nicotine in the blood, which allows to avoid using tobacco to replenish its "stock" in the body.
| Body system / Adverse Reaction | Bupropion HCl 300 mg/day (n = 376) | Bupropion HCl 400 mg/day (n = 114) | Placebo (n = 385) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Body (General) | |||||||||
| Headache | 26% | 25% | 23% | ||||||
| Infection | 8% | 9% | 6% | ||||||
| Abdominal pain | 3% | 9% | 2% | ||||||
| Asthenia | 2% | 4% | 2% | ||||||
| Chest pain | 3% | 4% | 1% | ||||||
| Pain | 2% | 3% | 2% | ||||||
| Fever | 1% | 2% | – | ||||||
| Cardiovascular | |||||||||
| Palpitation | 2% | 6% | 2% | ||||||
| Flushing | 1% | 4% | – | ||||||
| Migraine | 1% | 4% | 1% | ||||||
| Hot flashes | 1% | 3% | 1% | ||||||
| Digestive | |||||||||
| Dry mouth | 17% | 24% | 7% | ||||||
| Nausea | 13% | 18% | 8% | ||||||
| Constipation | 10% | 5% | 7% | ||||||
| Diarrhea | 5% | 7% | 6% | ||||||
| Anorexia | 5% | 3% | 2% | ||||||
| Vomiting | 4% | 2% | 2% | ||||||
| Dysphagia | 0% | 2% | 0% | ||||||
| Musculoskeletal | |||||||||
| Myalgia | 2% | 6% | 3% | ||||||
| Arthralgia | 1% | 4% | 1% | ||||||
| Arthritis | 0% | 2% | 0% | ||||||
| Twitch | 1% | 2% | – | ||||||
| Nervous system | |||||||||
| Insomnia | 11% | 16% | 6% | ||||||
| Dizziness | 7% | 11% | 5% | ||||||
| Agitation | 3% | 9% | 2% | ||||||
| Anxiety | 5% | 6% | 3% | ||||||
| Tremor | 6% | 3% | 1% | ||||||
| Nervousness | 5% | 3% | 3% | ||||||
| Somnolence | 2% | 3% | 2% | ||||||
| Irritability | 3% | 2% | 2% | ||||||
| Memory decreased | – | 3% | 1% | ||||||
| Paresthesia | 1% | 2% | 1% | ||||||
| Central nervous system stimulation | 2% | 1% | 1% | ||||||
| Respiratory | |||||||||
| Pharyngitis | 3% | 11% | 2% | ||||||
| Sinusitis | 3% | 1% | 2% | ||||||
| Increased cough | 1% | 2% | 1% | ||||||
| Skin | |||||||||
| Sweating | 6% | 5% | 2% | ||||||
| Rash | 5% | 4% | 1% | ||||||
| Pruritus | 2% | 4% | 2% | ||||||
| Urticaria | 2% | 1% | 0% | ||||||
| Special senses | |||||||||
| Tinnitus | 6% | 6% | 2% | ||||||
| Taste perversion | 2% | 4% | – | ||||||
| Blurred vision or diplopia | 3% | 2% | 2% | ||||||
| Urogenital | |||||||||
| Urinary frecuency | 2% | 5% | 2% | ||||||
| Urinary urgency | – | 2% | 0% | ||||||
| Vaginal hemorrhage | 0% | 2% | – | ||||||
| Urinary tract infection | 1% | 0% | – | ||||||
| * Adverse reactions that occurred in at least 1% of patients treated with either 300 or 400 mg/day of the sustained-release formulation of bupropion hydrochloride, but equally or more frequently in the placebo group, were: abnormal dreams, accidental injury, acne, appetite increased, back pain, bronchitis, dysmenorrhea, dyspepsia, flatulence, flu syndrome, hypertension, neck pain, respiratory disorder, rhinitis, and tooth disorder. | |||||||||
Alexander Markov, Head of Medical and Registration Department of GlaxoSmithKline Pharmaceuticals in Ukraine, emphasized that the company is interested in careful monitoring of adverse reactions of drugs, including Zyban, because it is responsible to doctors and patients for meeting high requirements to the effectiveness and safety of medicinal products. To date, no cases of side effects while taking Zyban have been registered in Ukraine. Unfortunately, some mass-media published misleading information that Zyban allegedly caused death of patients. In this regard, the representative office of GlaxoSmithKline Pharmaceuticals in Ukraine considered it necessary to distribute a special statement. In fact, there is no evidence that the use of Zyban for the treatment of nicotine addiction can cause a fatal outcome. Clinical studies conducted so far indicate that the drug is safe.
Cardiovascular disease was found to be the most common cause of death in patients taking Zyban. In those cases where it was possible to conduct retrospective analysis of medical records, along with the fact of tobacco smoking there were indications of the presence of cardiovascular disease or other pathology associated with the risk of their development (e.g., diabetes mellitus, hypercholesterolemia, etc.). There is no evidence that Zyban, as an adjunctive treatment for nicotine dependence, increases the risk of death compared to that of smokers who do not take the drug. Moreover, statistics suggest that the risk of death with Zyban does not exceed the average for the general population.
Thus, the cause of death in most cases was diseases caused by smoking, and not by taking Zyban.
Alexei Viktorov noted that there is no drug that does not cause adverse reactions. In 1980. The WHO General Assembly approved the main criteria that a drug should meet. For example, it must be effective, safe, and affordable for the patient. In fact, no matter how "miraculous" a drug may seem, its use has a downside. This is due to the fact that any drug is a foreign agent for the human body - a xenobiotic, with which all systems of the body interact. As a result, the drug, while exhibiting a therapeutic effect, causes certain undesirable reactions.
At the stage of clinical trials of a new drug, its effectiveness and safety are evaluated. Usually no more than 150 patients take part, but such studies allow the safety of a medicine to be established with a certain degree of certainty. But a comprehensive characterization of a drug can be obtained only by monitoring adverse reactions during its wide clinical use. In civilized countries of the world, including Ukraine, there is a special service of pharmacovigilance, which registers the cases of adverse reactions of medicines.
Zyban is on the world pharmaceutical market for about 15 years. It has proven to be an effective and safe medicine. By this time the State Pharmacological Centre of the Ministry of Health of Ukraine has not received a single report of any side effect caused by the use of Zyban. But in spite of this fact, physicians and pharmacists should draw patients' attention to expected side effects of this medicine and contraindications for its use.
The specialists speaking at the press conference were unanimous in their opinion that Zyban is an effective and safe remedy for nicotine addiction. The necessity of active promotion of this medicine to the Ukrainian pharmaceutical market was emphasized. The Ukrainian scientists expressed gratitude to GlaxoSmithKline company for the contribution into the tobacco smoking control and also highly appreciated its adherence to the ethical principles. One of the company's principles is to provide reliable information about all adverse drug reactions.
By: Dr. Alan David